BJ Srinivasa, Naik Radheshyam, Eriat Govind, S Gunasagar, S Smitha, Prabudesai Shilpa,
Naveen Krishnamoorthy
From the Department of Medical Oncology and Department of Pathology, HCG Bangalore Institute of Oncology, Bangalore, India.
Corresponding Author:
Dr. Srinivasa BJ
Email: research.hcg@gmail.com
Abstract
Primary rhabdomyosarcoma (RMS) of breast and RMS, metastatic to breast are rare entities with limited case reports in literature. Here, we report a case of primary rhabdomyosarcoma of breast in an adolescent girl which was metastatic at presentation. She presented with progressively increasing lump in right breast with difficulty in breathing. Histopathology was suggestive of solid variant of alveolar type/embryonal carcinoma (undifferentiated type). Immunohistochemical reports were positive for muscle markers Vimentin and Myf-4, making definitive diagnosis of RMS. Patient had fast progression of disease and died after first cycle of chemotherapy.
|
6go6ckt5b8|3000F7576AC3|Tab_Articles|Fulltext|0xf1ffe4ea02000000c301000001000800 6go6ckt5b5idvals|195 6go6ckt5b5idcol1|ID 6go6ckt5b5|2000F757Tab_Articles|Fulltext Introduction
Rhabdomyosarcoma (RMS) is common in children and adolescence. It commonly involves extremities, head and neck (orbit, nasopharnyx, middle ear and oral cavity) and genito-urinary tract. Various case reports of non muscle origin of RMS were reported in previous literatures. RMS arising from the breast and metastases to other parts of the body is uncommon and we are reporting this case of primary RMS of breast as it was mimicking invasive ductal carcinoma (IDC) clinically.
Case Report
A 19 year old girl, presented with lump in the right breast since one month. She had difficulty in breathing since 15 days and she was evaluated outside as having intraductal carcinoma of the right breast. On presentation, she had productive cough for 5 days accompanied by on and off fever. On examination, she was moderately built, Eastern Cooperative Oncology Group (ECOG) performance status was 2/5. Her breast examination revealed, 4x3 cm of hard mass in the upper and outer quadrant of her right breast with no abnormality in left breast. There were enlarged axillary and right supraclavicular lymph nodes with clinical evidence of right pleural effusion. Facial oedema, engorged neck veins and oedema of right upper limb suggestive of superior vena cava (SVC) obstruction were evident.
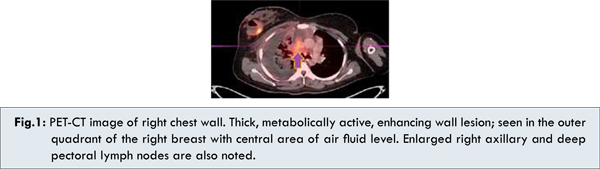
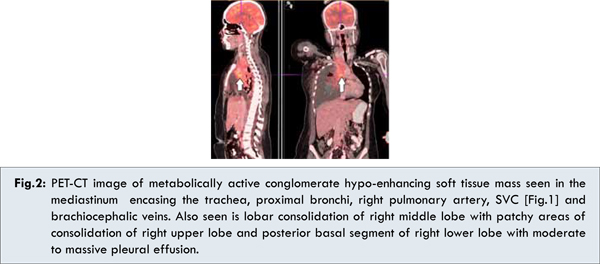
She was evaluated with Positron Emission Tomography - Computed Tomography (PET CT), which showed metabolically active, 4x4 cm of mass in the right breast with enlarged axillary lymph nodes, soft tissue mass seen in the mediastinum encasing the trachea, proximal bronchi, right pulmonary artery and SVC [Fig.1,2]. This was accompanied by consolidation of right middle lobe and multiple osteolytic lesions. Her biochemistry, uric acid and Lactate dehydrogenase (LDH), bone marrow aspiration was within normal limits. Pleural taping done to relieve her breathlessness was negative for malignancy.
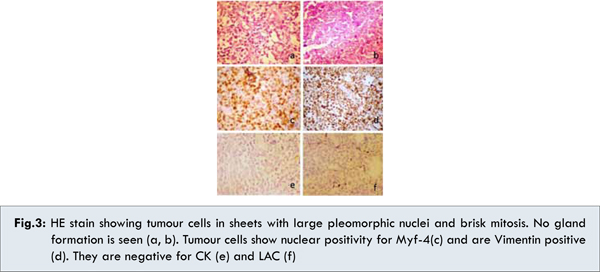
Histopathology of core biopsy of breast mass was suggestive of Rhabdomyosarcoma [solid variant of alveolar type/embryonal (undifferentiated) type] [Fig.3]. Immunohistochemical markers were positive for Vimentin, CD56, Myf-4 and was negative for CK, CD45, MPO, ALK1, CD20, CD3, CD10, Tdt, CD5, Synaptophysin, Chromogranin, CD99 and Melan A. We concluded that patient has primary rhabdomyosarcoma of the breast with metastases (IRS CLINICAL GROUP IV1).
She was planned on chemotherapy and has received her first cycle of chemotherapy with vincristine, actinomycin D and cyclophosphamide based regimen. She symptomatically improved with resolution of pleural effusions, with resolving signs of SVC obstruction. Follow up with family members revealed that on Day 5 post chemotherapy she suddenly collapsed, while getting up from the bed, and died on the way to the hospital.
 Discussion
Rhabdomyosarcoma (RMS) is thought to arise from immature mesenchymal cells that are committed to skeletal muscle lineage, but these tumours can also arise in tissues in which striated muscle is not normally found, such as in urinary bladder [1]. RMS comprise the most common single soft tissue sarcoma among children and adolescents, related to its linkage with somatic development and these tumours are uncommon in adults [2].
These tumours may arise from anywhere in the body, but depending on site of tumour, histology and age, few distinctions can be made. Embryonal variety occurs more commonly in head and neck region and in children less than eight years of age. Alveolar histology is predominant in adolescence, with extremity tumours. Botryoid variant is seen in younger children with genito urinary tract involvement. Among the other sites reported as case reports or series are intrathoracic, perineal- perianal region, biliary tract, liver, brain, trachea, heart, breast, or ovary [1]. Favourable sites are orbit, paratesticular and vagina while high risk sites are extremities and parameninges. Histologically alveolar variety has poorer outcome than embryonal varieties. Totally resectable tumour has better outcome while residual disease, metastatic disease has got bad prognosis.
Cell of origin of rhabdomyosarcoma is debated. Myogenic RMS may be due to subset of muscle forming cells called satellite cells. Non myogenic RMS may be due to mesenchymal progenitor cells which are committed not only to myogenic lineage but also produce tissue stromal elements (fat, fibroblasts, connective tissue). It is hypothesised that such cells may circulated in different organs and may give rise to RMS [3].
Evans reported primary rhabdomyosarcoma of breast in a 41 year old lady which was managed by mastectomy and patient had metastatic deposit to left upper arm and shoulder three and half year later, which was consistent as of primary in breast [4]. A case report [5] of 18 patients (16 females, two male) with breast cancer, two patients had primary (one RMS), 13 had metastatic disease (Nine had primary RMS elsewhere), three had secondary malignancy. All the patients with disease metastatic to breast died of the disease. IR study group reviewed 26 patients with RMS (seven primary, 19 metastatic) and compared with data regarding 47 similar patients in previously published reports, the histological subtype was alveolar in 24, embryonal in one, and not determined in one [6].
There are various case reports of RMS metastatic to breast from extremities [7]. Most of the patients reported so far had RMS, metastatic to breast from extremities, had alveolar RMS, had median age of about 15-20 years, treated with vincristine, cyclophosphamide, and dactinomycin based chemotherapy in metastatic setting and lumpectomy in primary only disease followed by adjuvant chemotherapy. Except in IRS case report (out of 19 three are disease free at 7.6, 15.7 and 17.0 years), most of the patient did not survive beyond six months after diagnosis. We have presented a 19 year old female with primary rhabdomyosarcoma of the right breast with extensive metastasis to lung and bone who was misdiagnosed as having IDC externally. Extensively evaluation here caused a delay in the diagnosis and treatment. She was treated with palliative chemotherapy with vincristine, cyclophosphamide and dactinomycin. She received 1st cycle of chemotherapy and expired post the 1st cycle.
Conclusion
Rhabdomyosarcoma of breast should be kept as one of differential diagnosis in adolescent females, unless strong family history of invasive ductal carcinoma of breast (BRACA1,2) is present. Diagnosis and treatment will be challenging, if careful clinical and histopathological correlation is not done.
Acknowledgement
HCG, Department of Medical Oncology and Stem cell and Bone Marrow Transplant and Anju Nidhin- Medical writer.
References
Discussion
Rhabdomyosarcoma (RMS) is thought to arise from immature mesenchymal cells that are committed to skeletal muscle lineage, but these tumours can also arise in tissues in which striated muscle is not normally found, such as in urinary bladder [1]. RMS comprise the most common single soft tissue sarcoma among children and adolescents, related to its linkage with somatic development and these tumours are uncommon in adults [2].
These tumours may arise from anywhere in the body, but depending on site of tumour, histology and age, few distinctions can be made. Embryonal variety occurs more commonly in head and neck region and in children less than eight years of age. Alveolar histology is predominant in adolescence, with extremity tumours. Botryoid variant is seen in younger children with genito urinary tract involvement. Among the other sites reported as case reports or series are intrathoracic, perineal- perianal region, biliary tract, liver, brain, trachea, heart, breast, or ovary [1]. Favourable sites are orbit, paratesticular and vagina while high risk sites are extremities and parameninges. Histologically alveolar variety has poorer outcome than embryonal varieties. Totally resectable tumour has better outcome while residual disease, metastatic disease has got bad prognosis.
Cell of origin of rhabdomyosarcoma is debated. Myogenic RMS may be due to subset of muscle forming cells called satellite cells. Non myogenic RMS may be due to mesenchymal progenitor cells which are committed not only to myogenic lineage but also produce tissue stromal elements (fat, fibroblasts, connective tissue). It is hypothesised that such cells may circulated in different organs and may give rise to RMS [3].
Evans reported primary rhabdomyosarcoma of breast in a 41 year old lady which was managed by mastectomy and patient had metastatic deposit to left upper arm and shoulder three and half year later, which was consistent as of primary in breast [4]. A case report [5] of 18 patients (16 females, two male) with breast cancer, two patients had primary (one RMS), 13 had metastatic disease (Nine had primary RMS elsewhere), three had secondary malignancy. All the patients with disease metastatic to breast died of the disease. IR study group reviewed 26 patients with RMS (seven primary, 19 metastatic) and compared with data regarding 47 similar patients in previously published reports, the histological subtype was alveolar in 24, embryonal in one, and not determined in one [6].
There are various case reports of RMS metastatic to breast from extremities [7]. Most of the patients reported so far had RMS, metastatic to breast from extremities, had alveolar RMS, had median age of about 15-20 years, treated with vincristine, cyclophosphamide, and dactinomycin based chemotherapy in metastatic setting and lumpectomy in primary only disease followed by adjuvant chemotherapy. Except in IRS case report (out of 19 three are disease free at 7.6, 15.7 and 17.0 years), most of the patient did not survive beyond six months after diagnosis. We have presented a 19 year old female with primary rhabdomyosarcoma of the right breast with extensive metastasis to lung and bone who was misdiagnosed as having IDC externally. Extensively evaluation here caused a delay in the diagnosis and treatment. She was treated with palliative chemotherapy with vincristine, cyclophosphamide and dactinomycin. She received 1st cycle of chemotherapy and expired post the 1st cycle.
Conclusion
Rhabdomyosarcoma of breast should be kept as one of differential diagnosis in adolescent females, unless strong family history of invasive ductal carcinoma of breast (BRACA1,2) is present. Diagnosis and treatment will be challenging, if careful clinical and histopathological correlation is not done.
Acknowledgement
HCG, Department of Medical Oncology and Stem cell and Bone Marrow Transplant and Anju Nidhin- Medical writer.
References
- Agarwala S. Pediatric Rhabdomyosarcoma and non rhabdomyosarcoma soft tissue sarcoma. J Indian Assoc Pediatr Surg. 2006;11:1.
- David M. Parham, Dale A. Ellison. Rhabdomyosarcomas in adults and children an Update. Arch Pathol Lab Med. 2006;130:1454-1465.
- Hettmer S, Wagers AJ. Muscling in: Uncovering the origins of rhabdomyosarcoma. Nature Medicine. 2010;16:171-173.
- Evans RW. Rhabdomyosarcoma of Breast. J Clin Pathol. 1953; 6:140–144.
- Rogers DA, Lobe TE, Rao BN, Fleming ID, Schropp KP, Pratt AS, et al. Breast malignancy in children. Journal of Paediatric Surgery. 1994;29:48-51.
- Hays DM, Donaldson SS, Shimada H, et al. Primary and metastatic rhabdomyosarcoma in the breast: neoplasm’s of adolescent females, a report from the Intergroup Rhabdomyosarcoma Study. Med Ped Onco. 1997;29:181-189.
- Keorochana G, Chanplakorn P, Laohacharoensombat W, Larbcharoensub N. Spinal and bilateral breast metastases of embryonal rhabdomyosarcoma. J Med Assoc Thai. 2007;90:813-818.
|