|
Parthasarathi Bhattacharyya Consultant, Institute of Pulmocare and Research, Kolkata, DG-8, New Town, Action Area-I, Kolkata 700156, India.
Corresponding Author:
Dr Parthasarathi Bhattacharyya Email: ipcr_india@yahoo.com
Abstract
Background: While varying degree of inflammation and fibrosis can mark a diffuse parenchymal lung disease (DPLD), no causative association has been forwarded between an often diffuse parenchymal infection as tuberculosis and DPLD. However, history of past tuberculosis has not been uncommon in DPLD. Case Report: A 48 years old non-smoker male presented with recently increasing shortness of breath persisting for eight years. He received partial treatment for diagnosis of strongly suspected pulmonary tuberculosis nine years ago. Spirometrically, he showed severe airflow limitation but radiologically he has several changes fitting to DPLD especially in the upper lobes with features of pan-acinar emphysema in lower lobes. There was no etiological clue for the detected DPLD like changes. A diagnosis of combined TOPD (tuberculosis associated obstructive pulmonary diseases) and ILD (interstitial lung disease) has been forwarded. Conclusion: It appears possible that tuberculosis may act as a causative factor for DPLD. This allows one to coin the term TILD (tuberculosis associated ILD). However, more evidence is required to establish this entity.
|
Introduction
Diffuse parenchymal lung disease (DPLD) is essentially a pro-fibrotic disease of the lung parenchyma with variable degree of inflammation and fibrosis. It is heterogeneous etiologically where no tangible cause can be ascertained occasionally and the situation is called ‘idiopathic interstitial pneumonia’. There is a huge (over 200) and growing list of conditions that leads to variable morphological descriptions fitting to DPLD with varied clinical presentation and outcome. For an index case, the etiological association of DPLD is established when a particular disease or event precedes the development without any other discernible cause. In this report, we describe such a patient in whom the past history of tuberculosis seems to be relevant to the causation of DPLD like morphological (HRCT chest) changes.
Case Report
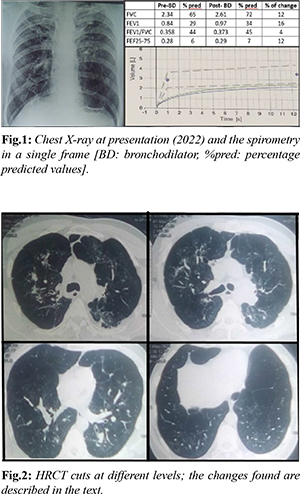
A 48-year-old male presented with history of shortness of breath for eight years that, of late, has increased and is now getting difficulty to climb up even one flight of stairs. He has been on regular use of inhalers (salbutamol-ipratropium followed by formoterol-budesonide combination) for having ‘bronchospasm’ noted by a physician. We sought and looked at the available past records. In 2013, the patient was given anti-tubercular therapy empirically on consent with strongly positive Mantoux test (28×30 mm), radiological (HRCT) suspicion of endo-bronchial dissemination of tuberculosis with sputum negativity for AFB (acid fast bacilli). As per the patient, he stopped the drugs for an overwhelming response after 2 months of regular medication. On examination, he was slightly malnourished (51 kg with height of 166 cm) with a pulse rate of 81 per minute and the SpO2 of 98% at rest in room air. The auscultation of the chest was normal. The chest X-ray revealed irregularly distributed GGO (ground glass opacities) with some small scars in both the upper zones and the para-hilar mid zones with the hila been pulled up. There are obvious airspaces in the lower zones especially on the left side. The cardiac shadow was found tubular with the diaphragm been somewhat flattened. The spirometry revealed FEV1 improving from 0.84 litres to 0.97 litres (35% of predicted) with the respective values of FEV1/FVC changing from 0.358 to 0.373 on bronchodilator to look for responsiveness. The pre and post bronchodilator Forced Expiratory Flow at 25-75% of FVC (FEF25-75) were 0.26 and 0.29 litres (merely 6% and 7% of predicted) respectively. The HRCT chest showed bilateral upper zone ground glass opacities restricted to central lung fields along with some lobular air-trappings [Fig.1]. There were some fine scarring, proximal bronchiectasis, and discrete clumps of alveolar densities [Fig.2]; the pulmonary vessels appeared dilated in multiple lobes. There was patchy pleural thickening especially in the postero-medial upper left thoracic cavity. In the lower lobes, scarring dominated below with pleural thickening mainly posteriorly. There were features of pan-acinar emphysema especially in the lower lobes. Thus, the HRCT picture was that of interstitial lung disease and emphysema together.
Discussion
The subject presented here met the GOLD diagnostic criteria for COPD both historically and spirometrically. Additionally, the presence of pan-acinar emphysema on HRCT chest confirmed the diagnosis of COPD. Given the history of tuberculosis with a response to anti-tubercular medication in the past and no history of smoking or chronic exposure to any known noxious substance, the patient could be diagnosed with TOPD (tuberculosis-associated obstructive pulmonary disease). The co-presence of possible fibrotic changes suggesting DPLD-features in both upper and lower zones in HRCT chest, supports the diagnosis of concomitant DPLD or ILD. Since the development of DPLD in this particular case can be traced etiologically down to the history of tuberculosis, it is possible that the so-called radiological changes of DPLD are also tuberculosis associated. Such a possibility is more obvious since the patient has not shown any features of any collagen vascular disease or had any significant exposure history. Hence, an expression as tuberculosis associated ILD or TILD (tuberculosis associated interstitial lung disease) may be appropriate here. Forwarding such a description of TOPD and TILD can evoke several questions here. TOPD is an already described reality. Allwood et al. elaborated it in 2014 [1] as an already recognized term where spirometry satisfying the definition of COPD in a non-smoker with history of tuberculosis in past can be designated as a case of TOPD. Out of several risk factors like smoking, biomass smoke exposure, chronic asthma, and air pollution, treated pulmonary tuberculosis is included as an important risk factor in the causation of COPD, particularly in developing countries [2,3]. Such post-tubercular development of COPD or TOPD was noticed and documented long back [4-7] and the entity has been confirmed by a systematic review by Allwood et al. in 2013 [8]. The index patient has been showing HRCT features of DPLD or ILD [Fig.2]. There is no evaluation of the common etiologies for DPLD, but the clear temporal association of tuberculosis and the observed ILD-like changes is obvious. Tuberculosis has several associations with ILD/DPLD. Mycobacterium tuberculosis has been grown more frequently from sputum of in ILD patients [9] and tuberculosis may occur more commonly in ILD [10]. Moreover, tuberculosis may mimic ILD [11], but tuberculosis causing ILD is hitherto not reported. Interestingly, it has been found that the history of tuberculosis is a definite risk factor for ILD [12]. While we cannot confirm the case as tuberculosis associated ILD, but definitely can keep the possibility in consideration. The future may reveal whether such a possibility exists.
Conclusion
This case suggests that tuberculosis may be an etiology for DPLD. Further evidence is needed to confirm this association.
Contributors: PB did manuscript writing and editing along with patient management. PB will act as a study guarantor and approved the final version of this manuscript and is responsible for all aspects of this study. Funding: None; Competing interests: None stated.
References - Allwood BW, Gillespie R, Galperin-Aizenberg M, Bateman M, Olckers H, Taborda-Barata L, et al. Mechanism of airflow obstruction in tuberculosis-associated obstructive pulmonary disease (TOPD). American Journal of Respiratory and Critical Care Medicine. 2014;189:A5832
- Zeng G, Sun B, Zhong N. Non-smoking-related chronic obstructive pulmonary disease: A neglected entity? Respirology. 2012;17(6):908-912.
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733-743.
- Hallett WY, Martin CJ. The diffuse obstructive pulmonary syndrome in a tuberculosis sanatorium. I. Etiologic factors. Ann Intern Med. 1961;54:1146-1155.
- Martin CJ, Hallett WY. The diffuse obstructive pulmonary syndrome in a tuberculosis sanatorium. II. Incidence and symptoms. Ann Intern Med. 1961;54:1156-1164.
- Birath G, Caro J, Malmberg R, Simonsson BG. Airways obstruction in pulmonary tuberculosis. Scandinavian Journal of Respiratory Diseases. 1966;47(1):27-36.
- Lancaster JF, Tomashefski JF. Tuberculosis a cause of emphysema. American Review of Respiratory Disease. 1963;87(3):435-437.
- Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86(1):76-85.
- Shachor Y, Schindler D, Siegal A, Lieberman D, Mikulski Y, Bruderman I. Increased incidence of pulmonary tuberculosis in chronic interstitial lung disease. Thorax. 1989;44(2):151-153.
- Chung MJ, Goo JM, Im JG. Pulmonary tuberculosis in patients with idiopathic pulmonary fibrosis. European Journal of Radiology. 2004;52(2):175-179.
- Akhter N, Rizvi NA. Interstitial lung diseases misdiagnosed as tuberculosis. Pakistan Journal of Medical Sciences. 2018;34(2):338.
- Choi WI, Dauti S, Kim HJ, Park SH, Park JS, Lee CW. Risk factors for interstitial lung disease: a 9-year Nationwide population-based study. BMC Pulmonary Medicine. 2018;18(1):1-7.
|